Dt&CRO
л©”лүҙм—ҙкё°
FDA Full Package Service
We dream of future through creative innovation and challenge
FDA GLP Study
Dt&CRO and Radyus Research have signed a Memorandum of Understanding (MOU) on March 15, 2024, to provide services covering
all stages from non-clinical trials to FDA approval, consultations for Investigational New Drug (IND) approval and new drug application (NDA).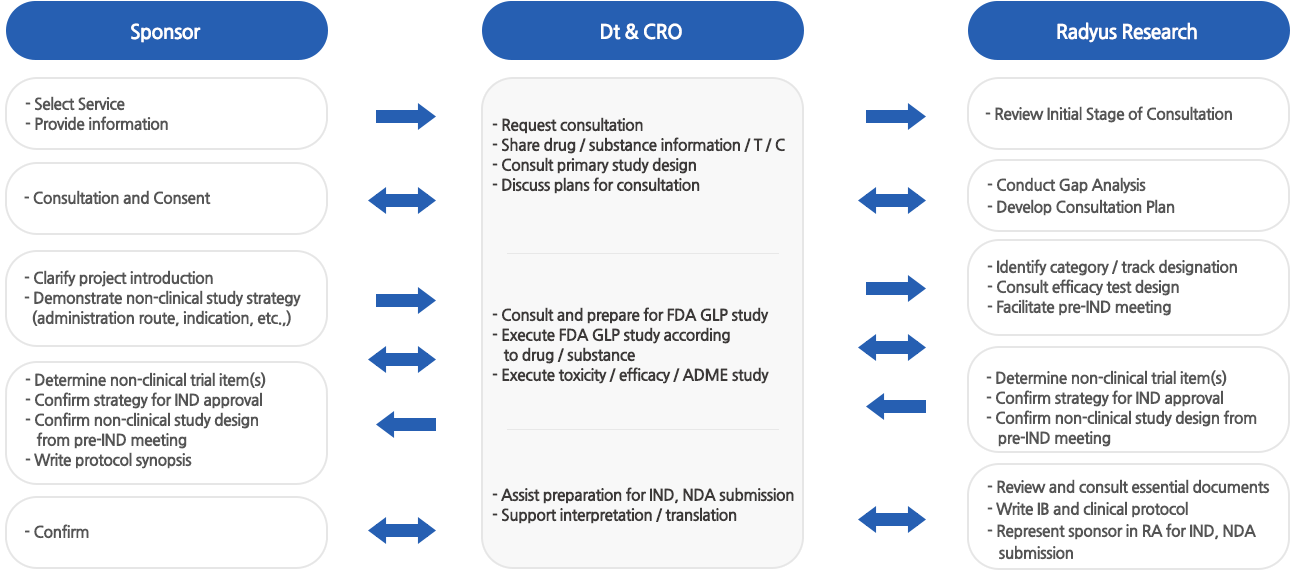
all stages from non-clinical trials to FDA approval, consultations for Investigational New Drug (IND) approval and new drug application (NDA).

Dt&CRO received its GLP certification in 2020. It is the only full-service provider of commissioned research in the country.
The company offers a complete range of services, including non-clinical GLP toxicity, PK,
and efficacy testing necessary for pre-approval of pharmaceuticals, chemical substances, health functional foods,
cosmetics, and medical devices. Additionally, Dt&CRO provides analysis, preclinical, clinical trial and pre-approval consulting services,
making it the top and only domestic provider of a complete CRO service in the Republic of Korea.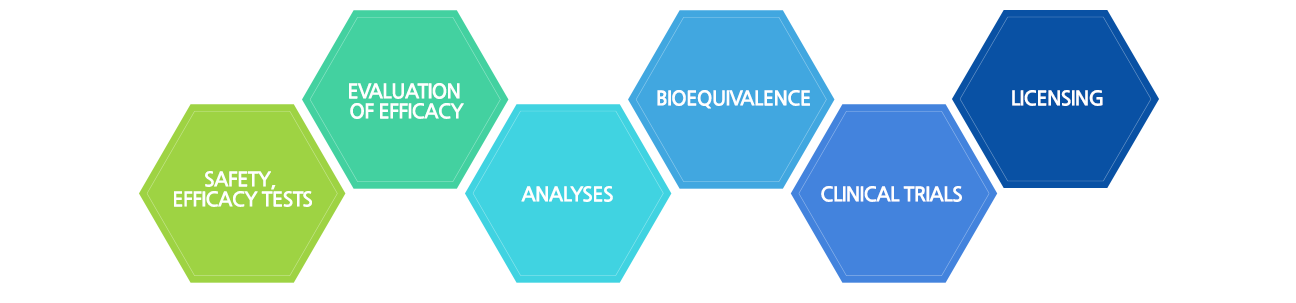
The company offers a complete range of services, including non-clinical GLP toxicity, PK,
and efficacy testing necessary for pre-approval of pharmaceuticals, chemical substances, health functional foods,
cosmetics, and medical devices. Additionally, Dt&CRO provides analysis, preclinical, clinical trial and pre-approval consulting services,
making it the top and only domestic provider of a complete CRO service in the Republic of Korea.
